






What’s this guide about anyway?
Back in 1998, I started my career as a medical device product development engineer.
At that time the FDA Design Controls regulations were still fairly new — not only to me — but the industry in general.
In those days, we all struggled to understand how and what to do with respect to Design Controls.
As my career progressed, I started to understand the purpose and
intent regarding Design Controls. But it didn’t happen overnight.
Over the past 17 years, I’ve been fortunate to have played a part in
getting 40+ medical devices through FDA clearance as a product
development engineer, through
my consultancy, and more recently as co-founder of
greenlight.guru.
However, for many, Design Control is still a topic that is as confusing today as it was for me many years ago.
With this guide, I plan to share valuable insights to explain
what Design Controls are,
how to address them, and
how they benefit your medical device product development efforts.
You have an idea for a new medical device. Now what?
Congratulations! You have an idea for a new medical device.
Chances are you believe your idea will have a significant impact on
the quality of our lives in some way. Chances are your product will help
solve some current problem and address unmet needs.
What do you do now?
Throughout the world, there are agencies that govern and regulate
medical devices. For example, in the United States, medical devices are
regulated by the Food & Drug Administration (FDA).
These regulatory agencies have defined rules and regulations that you
and others developing and manufacturing medical devices must follow.
And these regulatory agencies have defined rules and regulations
about how medical devices are classified and what is required before the
products are sold into the marketplace.
Most notably, since you have an idea that you want to develop
further, there are regulations established for you to follow during the
product development process. These regulations are known as Design
Controls.
This may sound confusing and discouraging. I get that.
And I don’t want you to be confused or discouraged.
This is what drove me to create this “Ultimate Guide to Design Controls for Medical Device Startups.”
In this guide, I will share with you the necessary background about Design Controls from a global regulatory perspective.
I will provide you with knowledge and information that will arm you with more than just the basics.

Some explanation about a Quality System
Yes, I know — this guide is supposed to focus on Design Controls. And it does.
I just need to spend a few minutes explaining what a quality system
is and how this relates to your medical device product development
efforts.
What is a Quality System?
A medical device company has to establish a quality system.
A quality system is a set of processes and procedures you define and
implement to describe how your company addresses medical device
regulations, including Design Controls.
FDA defines the rules in 21 CFR Part 820. And if you plan to go to market in the U.S., these regulations are required.
Outside the U.S., Europe requires a quality system be established to
meet the medical device directives. Many medical device companies choose
to implement a quality system and have it certified to ISO 13485 to
satisfy EU needs.
Canada — same thing. The expectation is that a quality system be
established. Canada is a little different, requiring you to establish
ISO 13485 certification
and the Canadian Medical Devices Conformity Assessment System (CMDCAS).
The good news is this…
FDA 21 CFR Part 820 and ISO 13485 are very similar. Meaning you can establish a “one size fits all” quality system, encompassing Design Controls too.
Build your Quality System as you go
The quality system expectations is that you have all parts and pieces defined and implemented by the time you go to market.
Yes, there are parts of the FDA regulations and ISO requirements that do apply to you, even if you are pre-market.
If you are going through medical device product development, there are
at least 4 parts of a quality system that you need to put in place:
- Design Controls
- Risk Management
- Document Control & Records Management
- Supplier Management
The term I use is “bootstrapping a QMS”. I encourage this approach.
Early on, you don’t need to spend too much time implementing a robust
quality system. You need to be focused on product development.
And as you get closer and closer to going to market, there are software tools, like
greenlight.guru and others, you can use to gradually implement more and more of a QMS.
Just make sure you always keep your quality system in mind from the beginning, so you don’t have to
learn how to free yourself from a quality system nightmare down the road.

What are Design Controls?
So far in this ultimate guide, I have spent very little time discussing Design Controls. Yes, this is deliberate.
The stuff I covered so far about understanding medical device product
classification and quality systems is very important for you to have
some grasp on as you pursue your new medical device idea.
If that information did not discourage you and you are still reading, rest assured.
The rest of this guide is devoted to Design Controls. Really more than just Design Controls.
I will share with you
why Design Controls even matter and how they will help you during your medical device product development.
Design Controls – Comparing FDA and ISO
It is important that you design and develop a medical device that is
safe. FDA, European Commission, Health Canada, and all other regulatory
bodies throughout the world will want some assurances that your medical
device is safe before you bring the product to market.
And
this is really the essence of Design Controls.
Proof that you have designed a safe product that meets user needs and requirements.
Technically speaking “Design Controls” is a FDA term and defined in FDA 21 CFR
820.30 (the 21 CFR stuff is FDA terminology to describe where in the code of federal regulations the topic is addressed).
In
ISO 13485 speak, the terminology and intent is similar and covered in section 7.3 Design and Development.
The table below compares the FDA clauses for Design Controls to ISO 13485 clauses regarding Design & Development.

Both FDA Design Controls regulations and ISO 13485 Design &
Development requirements expect you to keep documentation and records
throughout the product development process.
The Design History File (DHF) is a great place to keep all of your Design Controls “evidence”.
As you can see, there is consistency regarding Design Controls.
Although ISO 13485 does not explicitly call for a DHF, it is expected
that you maintain records of design and development.
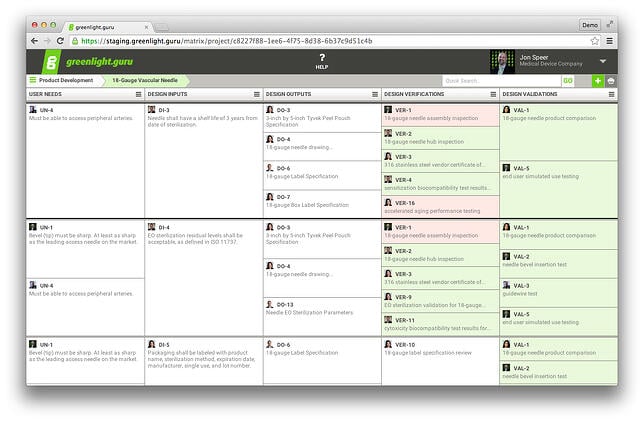
An industry best-practice is to construct a traceability matrix to
show the linkages and relationship between User Needs, Design Inputs,
Design Outputs, Design Verification, and Design Validation.
Building and maintaining your traceability matrix using tools like
Excel or Google Docs a is fairly straightforward task during the early
months of product development.
But as your project progresses, you’ll soon find using these general
purpose tools often take days, and in some cases weeks, to keep your
traceability matrix properly updated and maintained.
This marks a time in your project where switching to a software
solution built specifically to meet the unique regulatory needs of a
medical device company, like
greenlight.guru, can result in significant gains in time to market while reducing risk.
Product Development versus Design Controls
I’d like to spend a few minutes discussing product development versus
Design Controls. Is there a difference? Or are these terms synonymous?
The short answers to these questions depends on who you ask.
And I’m telling you the answers really don’t matter all that much.
When you have an idea for a medical device that you want to turn into
an actual product, you will follow some sort of product development
process. And I highly encourage you to map your product development
process and WRITE IT DOWN.
As an example, I like to define the product development process in major phases:

During product development, you will construct some prototypes, do
some testing, get some feedback, etc. Always trying to get to the next
step towards launching your medical device.
As noted above, Design Controls are all about ensuring the medical device you are developing
is safe.
All about making sure you have proven your medical device
meets the requirements you define.
All about making sure you have proven your medical device
meets the user needs and product’s intended use.
All about making sure you will be able to
manufacture your medical device that you designed and developed.
But since I posed the question about product development versus Design Controls, I’ll give you my opinion.
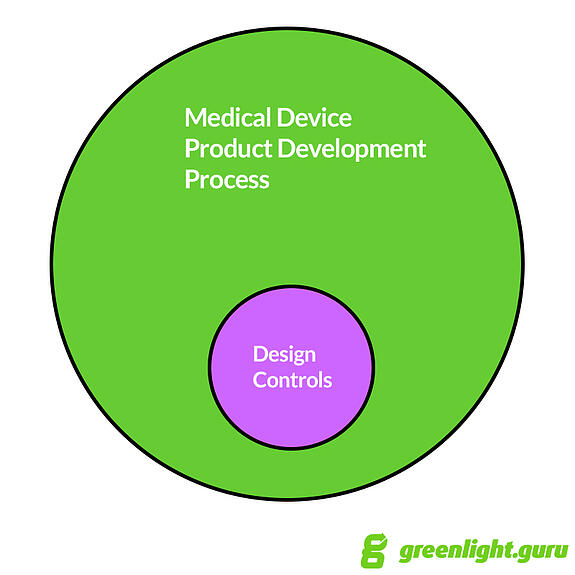
It’s my opinion that
Design Controls fits within the broader medical device product development process.
Product development includes much, much more than just Design
Controls. Things like budget, timeline, business development, marketing,
sales, and so on.

Maintaining a Design History File
What is a Design History File (DHF)? When you read FDA Design
Controls regulations, the part about DHF is mentioned towards the end.
And ISO 13485 doesn’t officially talk about a DHF.
I want to cover DHF now so that you understand what it is and why it matters.
A Design History File is, simply put, the place where you keep all your Design Controls.
A DHF should be organized and accessible to the entire project team.
A DHF is the place where you will show the linkages and relationships between all the Design Controls.
Demonstrating traceability of all your Design Controls throughout the product development process is expected and required.
Whether you use a paper-based approach, general purpose tools, or a
software solution designed specifically with Design Controls
traceability in mind like I mentioned before, know that
the responsibility to prove this is yours.
Keep the DHF separate from project file
My comments about product development versus Design Controls may seem somewhat trivial.
Except that it isn’t.
And the DHF is my main reason for saying so.
Yes, Design Controls and the DHF where these documents and records are maintained are important.
The DHF is the central hub for all the things medical device regulators care about.
Which is why it is so important to keep your Design History File as a stand-alone thing.
Going through medical device product development, you seldom consider how to maintain documentation and why it even matters.
And after you launch your medical device, the DHF is important in the event the project is ever
audited by the FDAor others.
The last thing you want to do when finishing up a project is to spend
weeks going back organizing documentation and records, including the
Design History File.
I’m telling you that if you do not keep things shipshape as you go, you will not go back to clean it up.
This is why I recommend building your Design Controls traceability
matrix early and keeping it up to date as you go. With this approach,
you won’t stress about the possibility of being audited because your DHF
will always be up to date and audit ready.
A Traceability Matrix Using greenlight.guru (Click here to get a free demo)
Ensure the DHF is organized and accessible
Ensuring Design Controls documentation and records are stored in a DHF is very important.
Keeping the DHF in a place where your entire product development
project team can access it is also important. Why? Throughout product
development, your team will be contributing valuable content to include
in the DHF, and accessing this same content to conduct product
development.
Be sure you are deliberate in how you plan to keep the DHF organized and accessible.
Do you plan to use a paper-based approach? If so, be sure you are aware of
the challenges paper systems pose.
Do you plan to store electronic records on a company server or some
type of electronic document management system? There are also challenges
with this approach as well.
Do your homework. Choose your solution wisely.

Why capturing User Needs sets the stage
Remember when you had the idea for your new medical device, you came
up with this wonderful product because you want to address unmet
clinical needs. There are two terms that you should understand:
intended use and indications for use.
Intended Use is the general purpose of the medical device or its function (what you “claim” the medical device does)
Indications for Use describe the disease or
condition the medical device will diagnose, treat, prevent, cure, or
mitigate, including a description of the target patient population
All of this relates to the User Needs for your medical device and are part of Design Controls.
Now you can do a lot of digging to try to get a better understanding
of what User Needs really are. FDA doesn’t really define User Needs in
the 820.30 regulations per se. User Needs are shown kicking off the
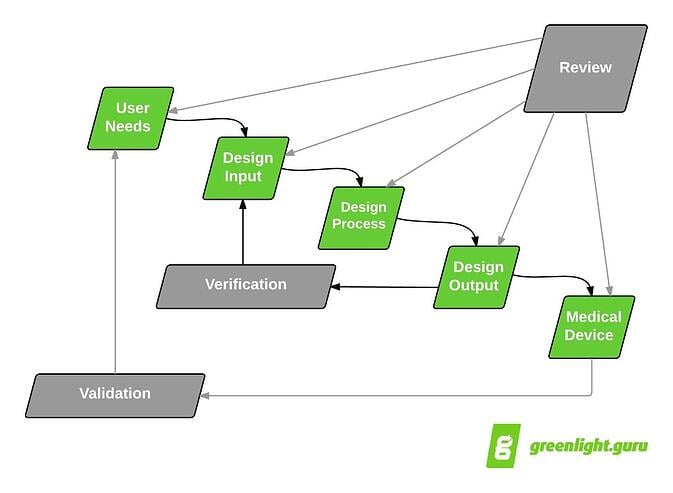
Design Controls process in the classic “waterfall diagram.”
Classic Design Controls waterfall diagram originally published by Health Canada & published in FDA Design Controls Guidance
The best place, aside from this guide, for you to get some insights about what User Needs are is the
FDA Design Controls Guidance.
And when you read the FDA guidance, just about every reference
involving User Needs is tied directly to Design Validation. Validation
proves your medical device meets User Needs and intended uses.
Documenting User Needs
Okay, so let me get into
documenting User Needs. When documenting User Needs, think about your medical device product idea.
User needs describe how your medical device is going to be used. User
needs help establish the framework for your medical device product
design. Intended use describes the clinical issue your product
addresses. Indications for Use pertain to clinical applications use,
environment, and end user.
Here are some questions to help you better understand User Needs.
- What do you want it to do?
- Who is going to use it?
- When will it be used?
- What important features and attributes should be considered?
- How will the user and patient interact with the device?
- What type of procedures will the device be used?
- What type of environment will the device be used?
- When will the device be used?
- Is the device used one time or over and over?
- What other products will the device interact and interface with?
There likely are dozens and dozens more questions you need to ask.
And answer! Document your responses to all the questions.
Your responses become the information you should consider as the User
Needs for your medical device idea, and included as part of your Design
Controls.
Looking again at the waterfall diagram, you see that the proposed
next major step after User Needs is Design Inputs. Logically, User Needs
are the lead into Design Inputs.
And many medical device product development professionals will share
with you how important Design Inputs are to the success of a new device.
Do not be too worried about whether or not your User Needs are
exactly correct. Do not get too hung up on the format and wording.
Do realize that one day in the future,
you will need to demonstrate that the product you develop addresses the User Needs you define.
The traceability matrix described earlier in this guide starts with
the User Needs. User Needs feed into Design Inputs. Design Validation
proves User Needs are met.

The purpose behind Design & Development Planning
In my experience, the topic of Design & Development Planning is
very misunderstood. When most medical device product developers think
about Design & Development Planning, the first thing that comes to
mind is this:
Project schedule.
Since there is confusion about this, let me share what FDA and ISO state about Design & Development Planning.
FDA 21 CFR 820.30(b) Design & Development Planning: Each
manufacturer shall establish and maintain plans that describe or
reference the design and development activities and define
responsibility for implementation. The plans shall identify and describe
the interfaces with different groups or activities that provide, or
result in, input to the design and development process. The plans shall
be reviewed, updated, and approved as design and development evolves.
ISO 13485:2003 7.3.1 Design and development planning: The organization shall establish documented procedures for design and development.
The organization shall plan and control the design and development of product.
During the design and development planning, the organization shall determine
a) the design and development stages,
b) the review, verification, validation, and design transfer
activities (see NOTE) that are appropriate at each design and
development stage, and
c) the responsibilities and authorities for design and development.
The organization shall manage the interfaces between different
groups involved in design and development to ensure effective
communication and clear assignment of responsibility.
Planning output shall be documented, and updated as appropriate, as the design and development progresses.
NOTE – Design transfer activities during the the design and
development process ensure that design and development outputs are
verified as suitable for manufacturing before becoming final production
specifications.
Did you come across anything that suggests project schedule? Or timeline?
Your product development schedule is definitely important. Don’t mishear me.
Remember where I shared some thoughts about product development versus Design Controls earlier in this guide?
Project schedule versus Design & Development Planning is in the same vein. Project
schedule is a product development thing. Yet there is also a definite
relationship with Design & Development Planning.
What goes into a Design & Development Plan?
Let me pick out the important aspects about Design & Development Planning from what FDA and ISO state about the topic:
- Identify Design & Development stages and activities
- Define responsibility for Design & Development activities
- Define the resources required; this includes project team members, as well as vendors
- Plans should evolve throughout Design & Development
A
Design & Development Plan describes
all the Design Controls, including when Design Reviews are expected.
The plan describes who the team members are, including roles and
responsibilities. The plan is not a “one and done” and needs to be
revisited and updated throughout the project.

Understanding the role and importance of Design Reviews
Design Reviews are moments in time for you to evaluate the progress
of your medical device project as it progresses through development.
During Design Reviews, you and the project team are formally reviewing — and agreeing to — Design Controls.
Design Reviews are also a time in a project to bring the team
together in order to scrutinize and evaluate the state of the medical
device and ensure that what needs to be done has been addressed.
How many? Who is involved?
How many Design Reviews does your medical device product development
project need? This is a good question to ask. I do not have the answer
for your project.
Here’s what I can tell you about
Design Reviews.
How many Design Reviews to have depends on numerous factors. Factors like
what the product is and
who the players are need to be considered when determining timing of Design Reviews.
The expectation is that your Design Review include the right functions (engineering, marketing, manufacturing, etc.). Also, you
need to include a resource who is independent.
When should Design Reviews happen?
All Design Controls must be included as part of a Design Review. As
noted, I cannot for certain tell you exactly when to have Design
Reviews. If you press me to provide a definitive response on this
question, I will defer to the what is suggested by the Design Controls
waterfall diagram.
I will advise you to have a Design Review
when User Needs are drafted.
I will advise you to have a Design Review
after Design Inputs are established.
And
after Design Outputs.
After Design Verification.
After Design Validation…and
before going to full production.
Design Reviews – the absolutes
Okay, let me be more helpful regarding Design Reviews.
First, you must have a record to prove that all Design Controls have been included as part of a Design Review.
Let me provide you a few “rules” when it comes to Design Review:
- Design Verification can not happen until Design Outputs and Design Inputs are done.
- Design Validation can not happen until User Needs are done.
- Design Transfer can not happen until Design Outputs are done.

Becoming a Design Input artist
Ah, Design Inputs. It is important for you to understand Design
Inputs and how critical these are to the entire success of your medical
device project.
Design Inputs, in my opinion, are the most important Design Controls.
Keep in mind that throughout medical device product development, the
terminology used is grossly inconsistent and highly dependent on who is
talking.
What I mean by this is that very few use the terminology as described
by FDA and ISO. Most have adapted their own lexicon when it comes to
medical device product development.
No where is this more true than on the topic of Design Inputs.
Keep this in mind as you define Design Inputs and work with others who will contribute.
Here is a non-inclusive list of terms that is often used to mean “Design Inputs”:
- Design Inputs
- Design & Development Inputs
- Design Input Requirements
- Requirements
- Design Requirements
- Product Requirements
- Requirements Specifications
It can be very confusing. And because of this, I highly encourage you
to make sure that you and your team members speak the same language
when it comes to Design Controls — especially Design Inputs.
What are Design Inputs?
Design Inputs define all the performance criteria, requirements, and features of your medical device product.
There are numerous resources that feed into Design Inputs. From a
Design Controls perspective, the primary resource that feeds Design
Inputs are the User Needs.
Often times User Needs are broad statements that may be difficult to
measure. User Needs include words like “easy”, “better”, “simple” —
subjective, abstract concepts. And this is okay. I encourage having User
Needs defined in this way.
- Design Inputs, though, should not be subjective, abstract concepts.
- Design Inputs should be objective. Measurable.
- Think of User Needs as the precursor to Design Inputs. (And show how these are linked in a traceability matrix.)
- Do not rely solely on User Needs as the only resource for Design Inputs.
There are things like industry standards, regulations, and
competitive products that you should also consider when it comes to
defining Design Inputs.
Plus, unless you are tackling medical device product development
solo, you have other people working with you who should help define
Design Inputs.
Think about Design Verification
I know I have not shared what Design Verification is just yet in this guide. Let me give you a preview:
Design Verification proves you designed the medical device correctly.
Because of this, you should definitely consider how you plan to
verify your product design at the time when you are documenting Design
Inputs.
Considering how you will verify will have a profound impact on the
content of Design Inputs. You should definitely put thought into how you
will prove your medical device will be verified when you define Design
Inputs.
Taking Design Verification into consideration when defining Design
Inputs will also help your product development efforts. This approach
will also help you plan and understand potential Design Verification
activities. This is meaningful because Design Verification tasks can be
very expensive and take significant amounts of time to complete.
Design Inputs become a roadmap for the product developer
Design Inputs describe everything that is important and required about your medical device.
Design Inputs become a roadmap, or set of “directions”, that a
medical device product developer uses to design and develop a product.
Design Inputs provide the important criteria that must be included in the design of the actual medical device.

Defining how to make your medical device through Design Outputs
Let’s check in a bit on what Design Controls I’ve covered with you so far.
I shared what a DHF is and why is matters. I explained Design & Development Planning. And importance of Design Reviews.
I talked about User Needs. About how User Needs are the precursor to Design Inputs.
Remember Design Inputs are the roadmap used to design and develop your medical device.
Let me spend a few minutes talking to you about Design Outputs being a
recipe and how these contribute to figuring out how to actually design
and develop your medical device.
Design Outputs are the recipe
As you get into the hands-on, nitty gritty design and development,
you start to identify all the parts and pieces that are required for
your medical device.
What components go into your medical device? How are these components put together?
What I’m trying to say is this:
Your medical device is
comprised of a number of materials, components, sub-assemblies, and so
on. And each of these parts need to be defined and documented.
Things like drawings, specifications, instructions.
Think of Design Outputs as the recipe for making your medical device.
Design Outputs are the documents you would give to someone to assemble
your product.
As you establish the Design Outputs, you need to show how these relate and link to the Design Inputs.
Once again, this can be done in Excel quite easily early on in
product development but as the project nears market release, these
relationships will become increasingly complex.
Design Outputs are the preliminary Device Master Record
Yes, this guide is about Design Controls. At the same time, it is
important how various Design Controls have an influence and impact after
you launch your medical device.
Design Outputs have a huge impact on the future state of your medical device. Fast forward with me for a moment.
When you have transferred from product development into
manufacturing, you need to establish a Device Master Record for each
product.
Device Master Record (DMR) is defined by FDA as:
a compilation of records containing the procedures and specifications for a finished device
A DMR is the recipe.
Just like Design Outputs.

Proving you designed correctly via Design Verification
Let me take you back a couple of topics. Think (or look) back at
Design Inputs. I expressed that there is a strong relationship with
Design Inputs and Design Verification. I encouraged you to think about
how you are going to conduct Design Verification when you define Design
Inputs.
When you get to Design Verification, your goal is to prove your
Design Outputs meet the Design Inputs. Design Verification is all about
demonstrating you correctly designed your medical device.
If you have been keeping your Design Controls traceability matrix up
to date, you have a clear picture of the relationship between your
Design Inputs and Design Outputs. Having this clear picture is very
helpful when it comes to conducting Design Verification.
So how do you conduct Design Verification?
Testing, Inspection, Analysis
When you ask an experienced medical device product developer to
describe Design Verification, it’s likely you will hear this described
as testing.
“Yes, testing is a very common method used to conduct Design Verification.”
Remember, Design Verification is about
proving Design Outputs meet Design Inputs.
Yes, testing is a completely valid way to prove this.
Although testing is
not the only way to conduct Design
Verification. You might also be able to employ inspection and analysis
as acceptable methods for Design Verification.
- Plan your Design Verification before you conduct tests, inspection, analyses.
- Identify what you are going to do before you do it. Determine the best ways to prove Design Outputs meet Design Inputs.
- And then do it! Complete your Design Verification activities, document results.
- Update your traceability matrix to show how Design Verification proves your Design Outputs meet Design Inputs.
Preparing regulatory submissions
I want to spend a few moments now talking a bit about regulatory submissions and the relationship with Design Controls.
Generally speaking, as you successfully finish Design Verification,
this means you are approaching a time when you can prepare a regulatory
submission.
Specifically, this is the time in your medical device project when you should put together your
FDA 510(k) submission (provided this is your path to market clearance).
If you plan to conduct a human clinical investigation in the U.S., this also marks the time when you should put together a
FDA investigational device exemption (IDE) submission. (An IDE is likely for
FDA PMA devices or could be of interest for clinical investigation before receiving 510(k) clearance.)
Outside the U.S. regulatory submissions and files are provided to regulatory bodies during Design Transfer.
Let me share a few links that are step-by-step examples for how to handle preparing different types of regulatory submissions.
CE Marking Process for Medical Devices – A Step by Step Example
Canadian Licence Process for Medical Devices – A Step by Step Example
FDA 510(k) or CE Marking?

Proving you have the correct device via Design Validation
Design Verification is about proving you designed your medical device
correctly(e.g.: the Design Output meets the Design Input requirements).
Design Validation is about proving you designed the
correct medical device.
Please understand the distinction. To do so, it helps to know that Design Validation demonstrates that your medical device meets User Needs and its intended uses.
Design Validation is the time when you revisit these User Needs. Validation is when you demonstrate that the User Needs are met.
Okay, not you per se. But actually end-users. Yes, that’s right —
end users should be involved with Design Validation.
This is the whole intent and purpose: prove your medical device meets the needs of the end user.
Design Transfer begins
Design Validation most definitely involves evaluation of products.
And because of this, the process of transferring a medical device from
product development to production (often referred to as “Design
Transfer”) begins during Design Validation.
In order to validate the design of your medical device, you need to
build products. These products are to be built using production
equivalent documentation and processes.
So all those Design Outputs (the preliminary DMR) need to be
converted to the production environment and used by production personnel
to assemble devices.
These production units (maybe the first) are then put in the hands of end users.
Have a plan of attack
If you remember the discussion about User Needs, I shared that
sometimes User Needs have a tendency to be a little ambiguous, using
words like “easy” and “better”. With Design Validation, you need to
figure out how to prove that your product accomplishes this.
You need to establish a Design Validation plan. This plan needs to identify how many end users, what type of testing is required, and so on.
The end goal of Design Validation is to have
objective, documented evidence.
Completing Design Validation is an important step in closing the loop
on your traceability matrix too. The traceability matrix started with
User Needs and ends with Design Validation.

Completing Design Transfer
As I noted, the Design Transfer process actually starts earlier in
your project. It begins when you build products for Design Validation.
I also shared how Design Outputs are the preliminary Device Master
Record. And the DMR is used by production to make medical devices.
Once you complete Design Validation, you are anxious to officially wrap up product development.
It’s time for you to have a final Design Review. This one may be a doozy (and may be a good idea to break it up in chunks).
The goal of this Design Transfer Design Review is to make sure everything that is needed for production is ready and done.
Completing Design Transfer signifies your medical device is ready to
exit product development and officially enter into production.
This is significant because up until this point the “control” of the
medical device has been the responsibility of the project team.
Once Design Transfer occurs, the control shifts to production resources.
Button up the DHF
I hope you are keeping your Design History File up to date and
current as you go through the medical device product development
process. This is the best option by far!
Nothing is worse than getting to the Design Transfer stage and
discovering that the DHF needs work. It happens. And if your DHF needs
work, take the time and do it.
Don’t fall into the trap of saying that you will get to it later.
You won’t.
I’ve shared throughout this guide tips and pointers regarding the
need to create and maintain a Design Controls traceability matrix.
Keeping your traceability matrix current and up to date makes Design
Transfer a much simpler process.
After you complete Design Transfer,
the DHF is the ultimate record proving you have satisfied Design Controls.
And your DHF might be reviewed, audited, and/or inspected one day.
Keep that in mind.

Launch!
After you complete your Design Transfer Design Review, you are almost
ready for market launch. You also need to ensure that you have all the
necessary regulatory clearances.
Then, at least from a Design Controls perspective, it’s time to sell your medical device.
The Ultimate Guide To Design Controls For Medical Device Startups

Summary of the Ultimate Guide to Design Controls for Medical Device Startups
Design Controls should be a systematic way to demonstrate the progress of your medical device product development efforts.
In this guide, I have shared with you what is expected for Design
History File, User Needs, Design & Development Planning, Design
Reviews, Design Inputs, Design Outputs, Design Verification, Design
Validation, and Design Transfer.
I hope this guide helps answer all your questions about Design
Controls (let me know in the comments!) — even the questions you didn’t
know you had.
Medical device product development should follow a structured,
methodical approach with traceability throughout. But we all know
nothing always goes according to plan.
That’s why in addition to this guide, we’ve also created
greenlight.guru,
an easy to use, cloud-based software platform designed specifically to
help medical device startups better managing their Design Controls &
Quality System.