Environmental, Health and Safety (EHS) Management and Audit

The practice of companies auditing their environmental, health and safety (EHS) began in the 1970’s, almost contemporaneously with the enactment of OSHA. Around that time, the environmental issue was gaining ground in the corporate circles of the West with the governments and other agencies pitching in with their efforts to create greater awareness of the impact of business activities on the environment. As a result, the thinking that the top management of an organization needs to be viewing this issue more seriously started to develop and got ingrained over the years.
 Cannot be glossed over
Cannot be glossed overAs a result of various legislations on the issue of environmental safety; the role of the Board of Governors became central in ensuring this aspect of the business. Environmental health and safety was no longer something that needed to be administered superficially, but in formal and designated ways, more specifically in the form of an audit. In order to incentivize corporate entities to implement environmental health and safety (EHS) management and audit; the trend started moving towards making these activities carry value addition to the organization.
Environmental health and safety management and audit is now a more formalized activity that needs to be carried out in a proper, set and well-defined manner. The processes that go into the EHS management and audit are clearly laid out in the form of standards such as the ISO 14001 standard, which is essentially an Environmental Management System (EMS) audit. To strengthen and enrich the audit activity and round it better; a few related and parallel standards such as the relevant parts of the 9000 family of standards, which deals with quality management, and 18000 series audits can be carried out with ease to supplement the environmental, health and safety audit.
 Role of environmental health and safety (EHS) management and audit
Role of environmental health and safety (EHS) management and auditEnvironmental health and safety (EHS) management and audit have now evolved into being a practice that is coupled with and fused into many business-related activities. The practice now is to make an environmental health and safety management and audit an inseparable part of the Quality Management System. Environmental health and safety (EHS) management and audit audits are now a sure means to ensure that the organization has a reputation for corporate social responsibility by implementing this audit.

Click to Continue Reading
Labels: EHS, Environmental, environmental health, Health and Safety, Management and Audit, safety management, safety management and audit
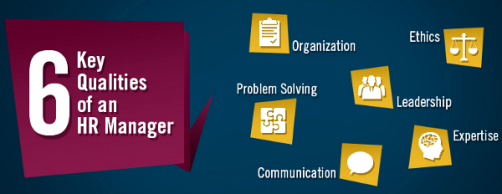
 There are many
There are many  Similarly,
the OSHA standards are meant to ensure that the workplace is free of
hazards. Depending on the kind of workplace, many OSHA provisions have
to be complied with. In short, there are a host of employment laws that
organizations have to comply with.
Similarly,
the OSHA standards are meant to ensure that the workplace is free of
hazards. Depending on the kind of workplace, many OSHA provisions have
to be complied with. In short, there are a host of employment laws that
organizations have to comply with. Then,
there should be processes for the implementation of the next set of
laws, those pertaining to employment conditions. When developing an
effective HR compliance program, anti-discrimination laws, laws on
sexual harassment, laws on dealing with violent employees or those who
have addiction problems, laws on workplace safety and many others need
be taken into consideration and carefully complied with.
Then,
there should be processes for the implementation of the next set of
laws, those pertaining to employment conditions. When developing an
effective HR compliance program, anti-discrimination laws, laws on
sexual harassment, laws on dealing with violent employees or those who
have addiction problems, laws on workplace safety and many others need
be taken into consideration and carefully complied with.
 So, what factors need to be taken into consideration when a
So, what factors need to be taken into consideration when a 





 A
country like India has a long way to go before it can build the kind of
infrastructure of the kind seen in the developed countries. Although it
has a huge network of railroads and roads; the quality is pathetically
low, affecting the efficiency of the supply chain. Delivery gets
affected in an environment of poor infrastructure and low implementation
of technology, although India is relatively stronger in the latter
area. Logistics and warehousing need to improve in a large measure if
the demand for growth in this sector has to be matched by the requisite
infrastructure.
A
country like India has a long way to go before it can build the kind of
infrastructure of the kind seen in the developed countries. Although it
has a huge network of railroads and roads; the quality is pathetically
low, affecting the efficiency of the supply chain. Delivery gets
affected in an environment of poor infrastructure and low implementation
of technology, although India is relatively stronger in the latter
area. Logistics and warehousing need to improve in a large measure if
the demand for growth in this sector has to be matched by the requisite
infrastructure.
 In the other “happening” countries – for the supply chain and
In the other “happening” countries – for the supply chain and 

 This
trend has its challenges, for sure, because the cost of making it work
could be unimaginably high. As with any new trend, it is going to be
some time before the economies of scale of such huge logistical
operations start kicking in. Yet, this is a very tangible factor that is
sure to count among the supply chain and logistics trends of 2017.
This
trend has its challenges, for sure, because the cost of making it work
could be unimaginably high. As with any new trend, it is going to be
some time before the economies of scale of such huge logistical
operations start kicking in. Yet, this is a very tangible factor that is
sure to count among the supply chain and logistics trends of 2017. As
a result of these trade and technological developments; governments all
around the world without exception are jumping on the bandwagon to
formulate laws that apply to the supply chain industry. For most
governments, global supply chain regulatory compliance is a must. They
are becoming extremely strict in enforcing these laws and are handing
out severe punishments to businesses and organizations that are lax in
enforcing global supply chain regulatory compliance. Most countries are
legislating laws regarding global supply chains almost exclusively, in a
way that other political acts are framed. This has pushed the need for
global supply chain regulatory compliance further.
As
a result of these trade and technological developments; governments all
around the world without exception are jumping on the bandwagon to
formulate laws that apply to the supply chain industry. For most
governments, global supply chain regulatory compliance is a must. They
are becoming extremely strict in enforcing these laws and are handing
out severe punishments to businesses and organizations that are lax in
enforcing global supply chain regulatory compliance. Most countries are
legislating laws regarding global supply chains almost exclusively, in a
way that other political acts are framed. This has pushed the need for
global supply chain regulatory compliance further. Other
developments in allied areas such as intellectual property rights have
gone on to strengthen the role of global supply chain
Other
developments in allied areas such as intellectual property rights have
gone on to strengthen the role of global supply chain 
 In
a nutshell, this U.S. Customs and Border Protection (CBP)-mandated
regulation aims to bring in place a single portal which will make
information on imported goods flow electronically from the businesses
themselves right to the respective departments or agencies that require
and handle them. The ACE is aimed at making import and export related
work paperless, while also seeking to become a major facilitator of
trade.
In
a nutshell, this U.S. Customs and Border Protection (CBP)-mandated
regulation aims to bring in place a single portal which will make
information on imported goods flow electronically from the businesses
themselves right to the respective departments or agencies that require
and handle them. The ACE is aimed at making import and export related
work paperless, while also seeking to become a major facilitator of
trade.
 There
is great diversity and complexity of laws that govern trade compliance
and logistics. They are now more integrated than at any point of time
before because of the advent of new technologies. This brings in a
motley mix of the
There
is great diversity and complexity of laws that govern trade compliance
and logistics. They are now more integrated than at any point of time
before because of the advent of new technologies. This brings in a
motley mix of the  For
many in the business of exports, complying with the global supply chain
may seem complex. However, knowledge of the laws of respective
countries and building a sound logistics and supply chain infrastructure
will go a long way in mitigating the problems associated with these.
This is
For
many in the business of exports, complying with the global supply chain
may seem complex. However, knowledge of the laws of respective
countries and building a sound logistics and supply chain infrastructure
will go a long way in mitigating the problems associated with these.
This is  Another
important reason for which trade compliance and logistics must work
together is that there are substantial long term gains to be had by
doing so. Supply chains that have come about as a result of trade
compliance and logistics working together become more reliable and
efficient. All these mean reduced costs in the long run, increased
customer satisfaction, and enhanced reputation in the business.
Another
important reason for which trade compliance and logistics must work
together is that there are substantial long term gains to be had by
doing so. Supply chains that have come about as a result of trade
compliance and logistics working together become more reliable and
efficient. All these mean reduced costs in the long run, increased
customer satisfaction, and enhanced reputation in the business.
 Another way of understanding logistics and supply chain management is this:
Another way of understanding logistics and supply chain management is this:

 A
sound supply chain system seeks to create value for the organization by
building and utilizing logistics infrastructure. Logistics and supply
chain management become meaningful when the organization synergizes
demand with supply, stock and supply and inventory management
A
sound supply chain system seeks to create value for the organization by
building and utilizing logistics infrastructure. Logistics and supply
chain management become meaningful when the organization synergizes
demand with supply, stock and supply and inventory management The
Foreign Corrupt Practices Act, or the FCPA, was enacted by the American
Congress in 1977. Its primary purpose is the prevention of bribing of
foreign officials to favor their business interests. In particular, the
anti-bribery provisions of the Foreign Corrupt Practices Act prohibit
using mails or other such means of communication to seek favors and
making payments of money or other articles of value with the intention
of obtaining favorable terms of business.
The
Foreign Corrupt Practices Act, or the FCPA, was enacted by the American
Congress in 1977. Its primary purpose is the prevention of bribing of
foreign officials to favor their business interests. In particular, the
anti-bribery provisions of the Foreign Corrupt Practices Act prohibit
using mails or other such means of communication to seek favors and
making payments of money or other articles of value with the intention
of obtaining favorable terms of business.

 Subject
to marketing approval by the respective regulatory bodies of the 25
developed nations; American companies can market their products to what
the FDA Export Reform and Enhancement Act of 1996 considers “Listed
Countries”:
Subject
to marketing approval by the respective regulatory bodies of the 25
developed nations; American companies can market their products to what
the FDA Export Reform and Enhancement Act of 1996 considers “Listed
Countries”: Far
from being frowned upon, complaints should serve as an opportunity for
medical device manufacturers to understand the customer’s expectations
better and lead to improvements in the product quality.
Far
from being frowned upon, complaints should serve as an opportunity for
medical device manufacturers to understand the customer’s expectations
better and lead to improvements in the product quality. Thirdly,
FDA 21 CFR Part 820 requires the manufacturer to establish and maintain
procedures for the receipt, review, and evaluation of complaints.
Thirdly,
FDA 21 CFR Part 820 requires the manufacturer to establish and maintain
procedures for the receipt, review, and evaluation of complaints.








 An indication of the extent to which the
An indication of the extent to which the  A
look at the history of the FDA points to the year 1848 as the start of
the first formal aspects of regulatory history. That was the year in
which Lewis Caleb Beck took his appointment with the Patent Office. His
mandate was to chemically analyze agricultural products. This is
considered as the first task that was aimed at regulating a product that
people consumed. This function rolled over to the Department of
Agriculture, which was created in 1862.
A
look at the history of the FDA points to the year 1848 as the start of
the first formal aspects of regulatory history. That was the year in
which Lewis Caleb Beck took his appointment with the Patent Office. His
mandate was to chemically analyze agricultural products. This is
considered as the first task that was aimed at regulating a product that
people consumed. This function rolled over to the Department of
Agriculture, which was created in 1862. President Franklin D. Roosevelt signing the 1938 Food, Drug, and Cosmetic Act.
Over time, the 1938 Act expanded to include more areas such as
cosmetics, devices and veterinary medicines, thus strengthening the
foundation for regulation and making it more expansive. Since the
passage of the 1938 Food, Drug, and Cosmetic Act; two major events
happened on the regulatory scene. The outbreak of tetanus and diphtheria
diseases in the 1960’s compelled the FDA to take a more proactive
approach to vaccinations.
President Franklin D. Roosevelt signing the 1938 Food, Drug, and Cosmetic Act.
Over time, the 1938 Act expanded to include more areas such as
cosmetics, devices and veterinary medicines, thus strengthening the
foundation for regulation and making it more expansive. Since the
passage of the 1938 Food, Drug, and Cosmetic Act; two major events
happened on the regulatory scene. The outbreak of tetanus and diphtheria
diseases in the 1960’s compelled the FDA to take a more proactive
approach to vaccinations.






 The
issuance of an FDA Warning Letter is an indication that the facility is
practicing some degree of nonconformity. A Warning Letter is among the
FDA’s strongest tools of ensuring voluntary compliance from
organizations with the provisions of the FDA Act.
The
issuance of an FDA Warning Letter is an indication that the facility is
practicing some degree of nonconformity. A Warning Letter is among the
FDA’s strongest tools of ensuring voluntary compliance from
organizations with the provisions of the FDA Act. A
General Warning Letter is one that is issued to a company in whose
activities the FDA notes significant variations from the principles laid
out in the FDA Act. The Warning Letter carries a description of the
variation or violation that the manufacturer has been practicing, along
with a description of what actions needs to be taken to correct it.
A
General Warning Letter is one that is issued to a company in whose
activities the FDA notes significant variations from the principles laid
out in the FDA Act. The Warning Letter carries a description of the
variation or violation that the manufacturer has been practicing, along
with a description of what actions needs to be taken to correct it. When
the FDA issues a Warning Letter to a facility that comes under the
various classifications; it follows up with it from time to time to
ensure that the suggestions it advises are carried out. When the
facility has carried out the necessary corrective actions; the FDA
issues the Warning Letter Closeout, which closes the matter associated
with the Warning Letter until the next FDA inspection.
Methods of issuing Warning LettersIt is only when it discovers violations that the
When
the FDA issues a Warning Letter to a facility that comes under the
various classifications; it follows up with it from time to time to
ensure that the suggestions it advises are carried out. When the
facility has carried out the necessary corrective actions; the FDA
issues the Warning Letter Closeout, which closes the matter associated
with the Warning Letter until the next FDA inspection.
Methods of issuing Warning LettersIt is only when it discovers violations that the  In
the perspective of the FDA; a Warning Letter is of an informal and
advisory nature. An FDA Warning Letter is a description of the violation
observed at a facility; but this in itself does not make the FDA take
enforcement action. Rather, through a Warning Letter, the FDA advices
the organization on what steps it has to take in order to rectify and
correct the reasons for which the Warning Letter was issued. An FDA
Warning Letter offers the organization enough opportunity to take
corrective action that is of a voluntary and appropriate.
In
the perspective of the FDA; a Warning Letter is of an informal and
advisory nature. An FDA Warning Letter is a description of the violation
observed at a facility; but this in itself does not make the FDA take
enforcement action. Rather, through a Warning Letter, the FDA advices
the organization on what steps it has to take in order to rectify and
correct the reasons for which the Warning Letter was issued. An FDA
Warning Letter offers the organization enough opportunity to take
corrective action that is of a voluntary and appropriate.